诚信认证:
工商注册信息已核实!

Halogen Bond: Leading Drug Design into a New Chapter
Sandra Forbes
Product Manager
In drug design, halogen bond interactions are often applied, but their conformation is mainly limited to the aromatic plane. Recently, Professor Franziska Zimmermann's team at the University of Tübingen in Germany published a paper in the journal J. Med. Chem., introducing the unique advantages of CF2X (X= Cl, Br or I) groups as halogen bond donors. This discovery provides a huge space for conformational innovation in the field of medicinal chemistry.
• Application of CF2X in Drug Molecular Design
By introducing CF2X groups into drug molecules, stronger non-covalent interactions can be formed with receptors, thereby enhancing the affinity and selectivity of drug molecules. This helps to improve the efficacy, metabolic stability, and pharmacokinetic properties of drugs.
The structure between Asciminib and ABL1 kinase mutants reveals the uniqueness of CF2X (X= Cl, Br or I) groups as halogen bond donors, forming XB interactions with receptors. This XB interaction opens up new possibilities in the field of drug design and medicinal chemistry, providing important insights for the development of new drug molecules with improved pharmacological properties.

Figure 1. XB interaction of Asciminib targeting the backbone oxygen of L448 in the myristoyl pocket of ABL1 (T334I_D382N) kinase (PDB ID: 5MO4). The sphere around the backbone carbonyl illustrates by color gradient the direction-dependent interaction energies calculated from using the (chlorodifluoromethoxy)benzene fragment of Asciminib at the MP2/TZVPP level of theory. Structural derivatives (13a, 13b, 13d, and 13e) of asciminib (13c) are presented herein.
• Application of CF2X in Chemical Biology Research
Compared to traditional alkyl halides, the two fluorine atoms in CF2X groups exhibit a strong negative charge effect, resulting in strong interactions with electron-rich receptor groups. This interaction alters the properties of bioactive molecules, enhancing their affinity and enabling better molecular recognition. The author's team has confirmed through systematic theoretical and experimental research that CF2X, as a halogen bond donor, has significant advantages compared to simple aryl halides. More importantly, due to the presence of two rotational degrees of freedom, CF2X can escape planar constraints and enter three-dimensional space, forming unique binding patterns with targets.

Figure 2. General molecular structures of Ph-X and Ph-Y-CF2X (X = H, F, Cl, Br, I; Y = O, NHCO) with dihedral angles (φ, ψ). The axis of rotation is defined as the middle of the three axes forming the dihedral angle.
• Application of CF2X in Protein Structure Studies
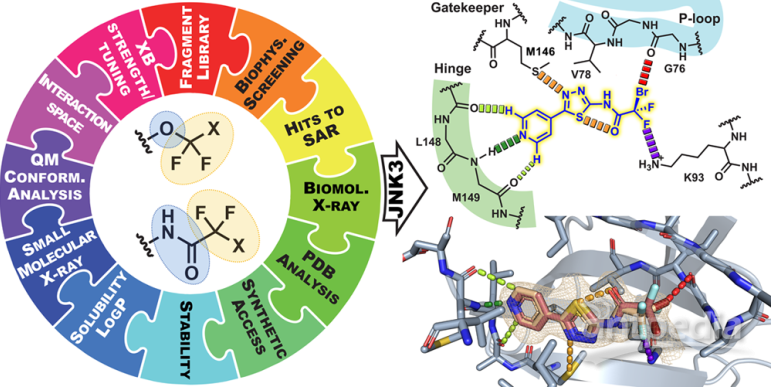
The research team introduced CF2X groups into a halogen-enriched fragment library and conducted biophysical screening to evaluate the binding capabilities of these fragments with JNK1 and JNK3 proteins. Ultimately, they successfully developed a novel JNK inhibitor that consists of only 18 carbon atoms. During binding, the iodine atom interacts with the target through a halogen bond, forming an unprecedented binding mode. This binding mode may involve the special properties of the halogen bond and precise targeting of protein active sites, providing new ideas and directions for drug design and development.
• Experimental Studies on CF2X Molecules
Using ether or amide linkers, it is possible to synthesize compounds containing CF2X molecules. These compounds are feasible to synthesize. In particular, amide derivatives are well-suited for fragment-based drug discovery. This provides the possibility of identifying non-traditional XB-based binding modes in biological targets.

Figure 3. 1-(4-(Difluorohalomethoxy)phenyl)urea (6a−e) and N-(benzo[d][1,3]dioxol-5-yl)-2,2-difluoro-2-haloacetamide (7a−e) derivatives for experimental studies.
The research on CF2X groups has opened a new perspective for conformational innovation in the field of medicinal chemistry. Traditional drug molecules are often limited to planar binding modes, while the application of CF2X groups provides the possibility of breaking through planar constraints in drug design. We expect that the creative application of this group will lead to the development of more unique candidate drugs, contributing to public health.
References
1. Sebastian Vaas, Markus O. Zimmermann, et al. Principles and Applications of CF2X Moieties as Unconventional Halogen Bond Donors in Medicinal Chemistry, Chemical Biology, and Drug Discovery. J. Med. Chem. 2023, 66, 10202−10225. https://doi.org/10.1021/acs.jmedchem.3c00634
https://www.aladdinsci.com/
